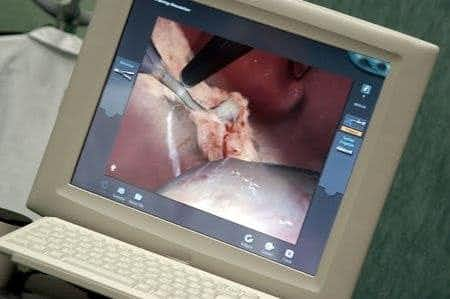
This case takes place in Colorado and involves patient who underwent a hysterectomy using a Da Vinci Surgical System. The patient suffered thermal rectal burn/perforation that was unrecognized during the procedure. She subsequently developed a range of issues, necessitating multiple corrective procedures and ongoing care, including high levels of chronic pain. It is alleged that the device is defectively designed/manufactured, and that the plaintiff would have had a better outcome with a more traditional laparoscopic procedure
Question(s) For Expert Witness
1. Please describe your experience/knowledge as it pertains to the Da Vinci surgical system, specifically as it relates to patients like the one in this case.
2. Have you ever served as an expert on a similar case? If so, please explain.
3. Are you familiar with the way that Intuitive Surgical sells the Da Vinci system to hospitals and physicians, as well as any training/certification provided to physicians using this system?
Expert Witness Response E-004882
I have been following the IS/daVinci saga for over 5 years with interest, as the multiple issues and alleged failures directly match up with my specific areas of expertise (electrical engineering/biophysics, software engineering, quality engineering & FDA regulations, and human factors/ergonomic engineering). I am also familiar with their FDA recalls, including the most recent ones just this year. I am working with a firm on the shareholder suit against Intuitive. I have worked as an expert witness on a number of medical device product liability cases, including very large MDLs, grouped cases in State courts, and individual plaintiffs' claims. I am familiar with (and have been following for the past few years) the public domain descriptions and allegations that IS labeling and training procedures violate multiple basic principles of human factors engineering and FDA regulations, as well as trying to shift the burden of training proficiency to the hospitals. As I have recently published, medical manufacturers can deem risks acceptable, but they can only accept those risks for themselves, not others (such as hospitals, providers, and patients).
About the author
Joseph O'Neill
Joe has extensive experience in online journalism and technical writing across a range of legal topics, including personal injury, meidcal malpractice, mass torts, consumer litigation, commercial litigation, and more. Joe spent close to six years working at Expert Institute, finishing up his role here as Director of Marketing. He has considerable knowledge across an array of legal topics pertaining to expert witnesses. Currently, Joe servces as Owner and Demand Generation Consultant at LightSail Consulting.